GlaxoSmithKline embroiled in scandal
Vivian Stamp | 12.04.2004 01:22 | Bio-technology | Health | Social Struggles | London
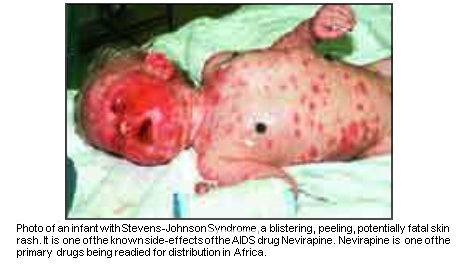
SHOCKING
 http://observer.guardian.co.uk/international/story/0,6903,1185305,00.html
http://observer.guardian.co.uk/international/story/0,6903,1185305,00.html UK firm tried HIV drug on orphans
GlaxoSmithKline embroiled in scandal in which babies and children were allegedly used as 'laboratory animals'
Antony Barnett in New York
Sunday April 4, 2004
The Observer
Orphans and babies as young as three months old have been used as guinea pigs in potentially dangerous medical experiments sponsored by pharmaceutical companies, an Observer investigation has revealed.
British drug giant GlaxoSmithKline is embroiled in the scandal. The firm sponsored experiments on the children from Incarnation Children's Centre, a New York care home that specialises in treating HIV sufferers and is run by Catholic charities.
The children had either been infected with HIV or born to HIV-positive mothers. Their parents were dead, untraceable or deemed unfit to look after them.
According to documents obtained by The Observer, Glaxo has sponsored at least four medical trials since 1995 using Hispanic and black children at Incarnation. The documents give details of all clinical trials in the US and reveal the experiments sponsored by Glaxo were designed to test the 'safety and tolerance' of Aids medications, some of which have potentially dangerous side effects. Glaxo manufactures a number of drugs designed to treat HIV, including AZT.
Normally trials on children would require parental consent but, as the infants are in care, New York's authorities hold that role.
The city health department has launched an investigation into claims that more than 100 children at Incarnation were used in 36 experiments - at least four co-sponsored by Glaxo. Some of these trials were designed to test the 'toxicity' of Aids medications. One involved giving children as young as four a high-dosage cocktail of seven drugs at one time. Another looked at the reaction in six-month-old babies to a double dose of measles vaccine.
Most experiments were funded by federal agencies like the National Institute of Allergy and Infectious Diseases. Until now Glaxo's role had not emerged.
In 1997 an experiment co-sponsored by Glaxo used children from Incarnation to 'obtain tolerance, safety and pharmacokinetic' data for Herpes drugs. In a more recent experiment, the children were used to test AZT. A third experiment sponsored by Glaxo and US drug firm Pfizer investigated the 'long-term safety' of anti-bacterial drugs on three-month-old babies.
The medical establishment has defended the trials arguing they enabled these children to obtain state-of-the-art therapy they would otherwise not have received for potentially fatal illnesses.
However, health campaigners argue there is a difference between providing the latest drugs and experimentation. They claim many of the experiments were 'phase 1 trials' - among the most risky - and that HIV tests for babies were not a reliable indicator of actual infection and therefore toxic drugs could have been given to healthy infants. HIV drugs are similar to those used in chemotherapy and can have serious side-effects.
Vera Sharav, president of the Alliance for Human Research Protection, said the children had been treated like 'laboratory animals'.
'These are some of the most vulnerable individuals in the country and there appears to be a policy of giving drug firms access to them,' she said. 'Throughout the history of medical research we have seen prisoners abused, the mentally ill abused and now poor kids in a care home.'
Sharav has urged the US Food and Drug Administration to investigate and has demanded full disclosure of all adverse effects suffered by the children, including deaths. Brooklyn Democrat councillor Bill de Blasio is also demanding that New York's Administration for Children's Services, which approved the trials, reveal who gave consent and on what grounds.
Glaxo has confirmed it provided funds for some of the experiments but denied any improper action. A spokeswoman said: 'These studies were implemented by the US Aids Clinical Trial Group, a clinical research network paid for by the National Institutes of Health. Glaxo's involvement in such studies would have been to provide study drugs or funding but we would have no interactions with the patients.
'Generally speaking, clinical research is carefully regulated in the US and it would be the responsibility of the appropriate authorities to ensure all subjects in a clinical trial provided appropriate, informed consent to conform with all local laws and regulations regarding legal authority in the case of minors.'
The Incarnation trials were run by Columbia University Medical Centre doctors. Columbia spokeswoman Annie Bayne said there had been no clinical trials at Incarnation since 2000 and that consent for the children was provided by the Administration for Children's Services, which uses a panel of doctors and lawyers to determine whether the benefits of a trial for each child outweighs the risks. 'There are many safeguards in the system. HIV is eventually a fatal disease, but drug therapy has lengthened life significantly,' said Bayne.
A spokesman for Incarnation said: 'The purpose of the trials was to test the efficacy of HIV medication ... These trials were based on scientific evidence of their potential value in the treatment of HIV-infected children.'
Vivian Stamp
Comments
Display the following 4 comments